With every breath you take, oxygen from the air rushes into your lungs, is absorbed into the hemoglobin in your blood and pumped by your heart throughout the trillions of cells in your body. If you doubt the importance of this, you can perform the simple experiment of holding your breath for a minute; as you turn blue, you will entirely convince yourself that oxygen is vitally needed by the cells in your body.
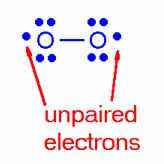
What do your cells do with all these oxygen molecules? Oxygen molecules are used by the cells to capture electrons from other molecules. Oxygen molecules have two unpaired electrons (called free radicals) that are hungry for companions. These oxygen free radicals capture available electrons from other molecules; when electrons are captured this is called “reduction”; oxygen is said to be “reduced” when it captures electrons. These electrons are stolen from other molecules; when electrons are ripped off of these molecules, this is called “oxidation”, these other molecules are said to be “oxidized”. Thus oxygen “oxidizes” other molecules by stealing their electrons and becomes “reduced” at the same time, by capturing such electrons. The special name REDOX (REDuction/OXidation) is given to this process.
Why is this REDOX process so important to cells? The metabolism of sugars, fats and keytones takes place within hundreds of power-generating mitochondria (my-toe-con-dree-ah) inside the cells. These mitochondria produce the energy needed to power our cells. Oxygen is needed as part of the final step of metabolism inside the mitochondria to capture the electrons that participate in the electron transport chain (ETC) (electrons are quickly transported to “charge the batteries” of the mitochondria). If oxygen were not there to capture these electrons, they would quickly leak back in, discharge the mitochondrial batteries, and in turn would cause our cells to run out of fuel and energy. Thus oxygen is vital to stop the electrons from leaking back, to keep these cell batteries charged, and to keep the cell’s machinery
working. More than 80% of the oxygen we breathe is used in the mitochondria for this purpose.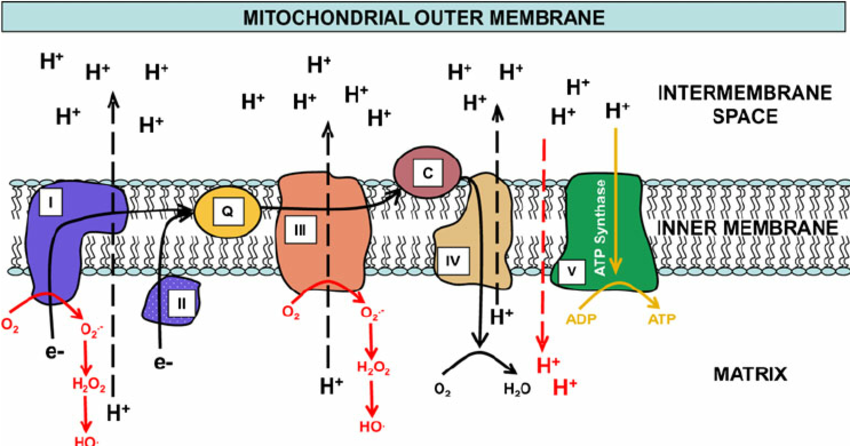
When an oxygen molecule (O2) captures an electron, it transforms into a new type of reactive oxygen called the “superoxide anion” free radical (O2*-). This type of oxygen radical is highly reactive. An enzyme called “superoxide dismutase” (an antioxidant made inside the cell) donates yet another electron to the superoxide and transforms it into a “peroxide”. This peroxide state of oxygen attracts two hydrogen atoms to become hydrogen peroxide (H2O2). From here, other enzymes in the cell (such as “catalase” and “glutathione”) again reduce this type of oxygen into its most reduced state, “water” (H2O). There are several other transformations that reduce oxygen, one of them combines oxygen with the chloride ion (from salt) to form the “hypochlorite anion” (OCl-). We will not mention any more of them here for simplicity’s sake.
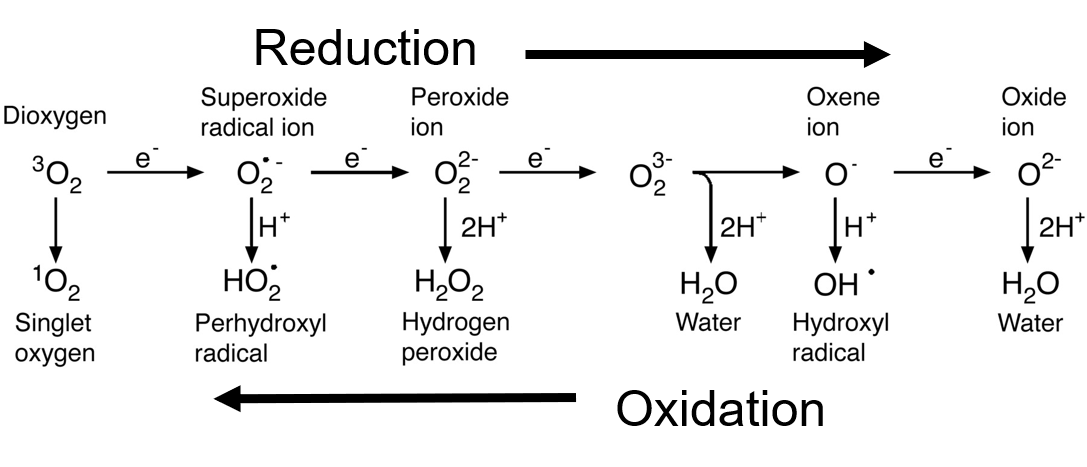
And so we see that the story of the oxygen molecule inside our cells involves a series of transformations as it is reduced from oxygen gas to water and the other states of oxygen. The types of molecules that are produced in these transformations (O2*-, H2O2, OCl-), as mentioned above, are given the name “reactive oxygen species” (ROS). These also are part of a group known as “redox signaling molecules”.
This story of how oxygen is used and transformed inside cells is as old as aerobic life on earth. Even earlier in the earth’s story, green plants on earth, through photosynthesis, were able to use the energy from the sun to run these oxygen transformations in reverse. Plants start by using sunlight to “bump” electrons off the plentiful oxygen atoms in the water molecules (H2O) to start the reverse transformation. In the plant cell, when an electron is removed from oxygen in the water molecule, the oxygen is transformed into various forms of ROS. Finally (after all the reverse transformations) the oxygen is transformed into the oxygen gas molecule (O2). This oxygen gas is released by the plants into the atmosphere. Thanks to plants, about 20% of the air in our atmosphere is now oxygen (O2). The story of how oxygen transforms from water to oxygen gas in plants, is released into the atmosphere, and then is transformed from oxygen gas back into water in animals is a vital part of the story of life, as we know it.
Only in the past decade have scientists realized the true importance of the intermediate forms of oxygen (ROS) in this transformation and the role they play inside living cells. We have found that these ROS forms serve as fundamental redox signaling molecules inside the cell. In short, they turn off and on the genetic switches that control what happens inside the cell, they are used to move electrons around inside the vital salt-water fluids that fill the cell. They cause transformations in the protein-based machinery of the cell that allow it to move (fold, unfold, change shape, break apart, come together, etc.), they serve to inform the cell that something is wrong when too many of them accumulate (a condition called oxidative stress). Depending on how many of them are in the cell, key functions of the cell are regulated. For example, too much hydrogen peroxide (H2O2) in plant cells will slow down photosynthesis (this keeps plants from “burning out” in full sunlight) and in animals, too much H2O2 will slow down the metabolism (this also prevents animal cells from “burning out”). These redox signaling mechanisms act like the controlling “gas pedal” in your car, they control the speed of the “motor” in the cell and keep it from “burning out”. In short, without these redox signaling molecules, it would be impossible for the cells to live, breathe and regulate themselves.
A knowledge of how these most universal and fundamental processes in life work gives us great power. As these oxygen transformations and the resultant redox signaling takes place in most every cell on earth, the reach of redox signaling technologies is almost universal. These redox signaling molecules effectively kill primitive bacteria (they are the primary weapon of the immune system), they turn on the alarms and repair genes inside cells, they participate in energy regulation (as already mentioned), they participate in electrical and chemical signaling networks everywhere. They are also the primary actors in the networks that recognize and kill defective cells and regenerate healthy cells. They are part of the networks that allow our cells, tissues, organs, and systems to cooperate and function together. Their life-saving potential is well known, we all know that administering oxygen can save lives. No longer are these reactive oxygen species (ROS) thought of as enemies, they are redox signaling molecules, a vital part of the primary tool chest of life. We can use them to live, and to live more abundantly.